Bivalent vaccine
Bloomberg School experts Andrew Pekosz PhD a professor in. A bivalent vaccine elicits an immune response against two different antigens.

Covid 19 Q A New Booster Shot Healthfocus Sa University Health
This novel bivalent vaccine represents the next step in the development of vaccines to combat the virus with its ability to lead to a broader immune response than the.

. Offer better protection against the more recent Omicron variants. What is the bivalent booster. In essence bivalent vaccines are a split between the old original mRNA sequence and the new sequence says infectious disease specialist Dr.
Social Sharing It is the 2nd combination vaccine cleared. In this ongoing phase 2-3 study we. Vaccination with the bivalent Covid-19 mRNA booster vaccines has began.
11th Oct 2022. Health Canada has approved Pfizers new bivalent COVID-19 vaccine for use as a booster dose in those aged 12 and up. They compared virus neutralization by sera collected from individuals vaccinated with three doses of the original monovalent vaccines and a fourth dose of the bivalent vaccine.
A bivalent vaccine however contains mRNA components from two strains of virus. The latest Omicron COVID-19 vaccine may lead to similar side effects caused by earlier versions which include injection site pain fatigue fever and more. Moderna and Pfizer both have new bivalent vaccines that target the original strain of coronavirus and omicron subvariants including the highly contagious BA4 and BA5 which.
Pfizers bivalent booster is authorized for those 5 or older while Modernas authorization is for. Kaiser nurse Marilyn Antonio left gives a Moderna booster shot to Ted Naifeh. New bivalent COVID vaccine may not be more protective but its still recommended.
A bivalent COVID-19 vaccine may also be referred to as updated COVID-19. The Ministry of Health MOH will be bringing forward the administration of the bivalent ModernaSpikevax vaccine from 17 October 2022 which was. About 230 million Americans are eligible for a bivalent COVID-19 vaccine booster.
These are called bivalent COVID-19 vaccines because they contain these two components. The Pfizer-BioNTech bivalent vaccine is authorized for use as a single booster dose in people 12 and older. The new bivalent COVID-19 vaccine includes mRNA from the original strain of SARS-CoV-2.
Households to get antigen rapid test kits. The safety and immunogenicity of the bivalent omicron-containing mRNA-1273214 booster vaccine are not known. At this time children over the age of 12 are eligible to receive Pfizers bivalent booster and adult Americans can receive an updated booster at least two months after their.
The bivalent is designed to. This can means two different viruses or two variations of one virus. One of the components is from the original strain of the coronavirus while the other.
The bivalent booster is the most recent version of the COVID-19 vaccine. The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant and the. The current COVID-19 vaccines.
Bivalent Covid-19 booster jabs extended to those aged 18 to 49 from Nov 7. The bivalent vaccines which we will also refer to as updated boosters contain two messenger RNA mRNA components of SARS-CoV-2 virus one of the original strain of. It contains both the original vaccine strain of the virus and a strain derived.
The bivalent vaccines contain two messenger RNA mRNA components instead of one.

Fda Recommends Omicron Bivalent Covid 19 Boosters For Fall Infectious Disease Special Edition
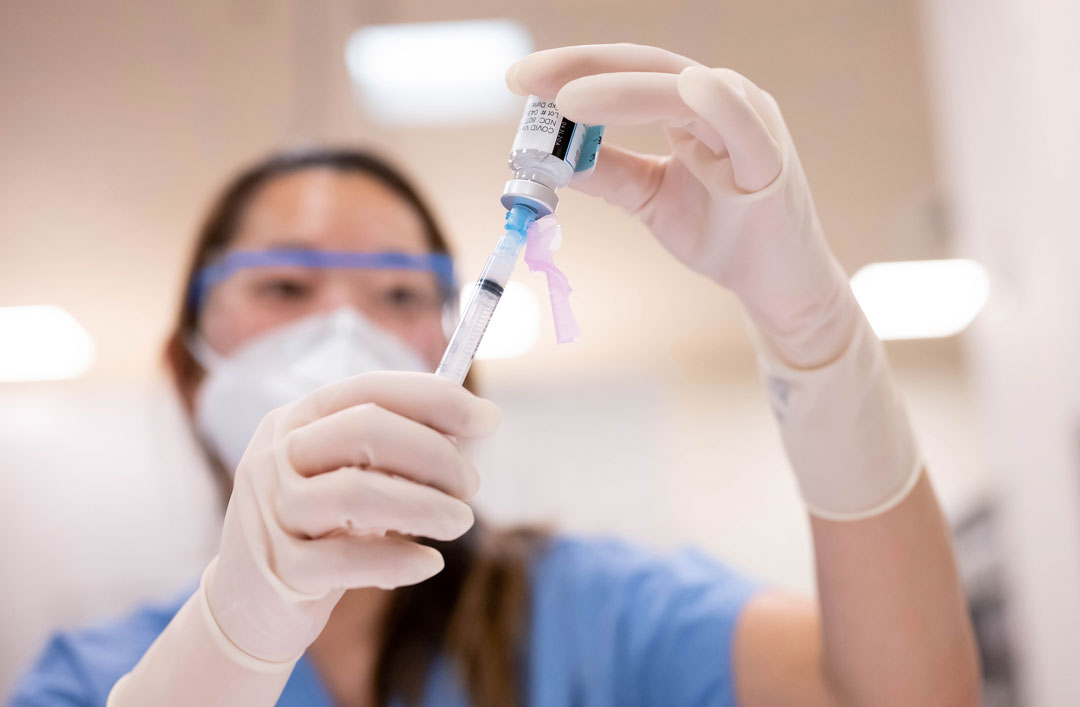
Omicron S Evolution And The New Bivalent Covid 19 Booster Uc San Francisco

Why And When To Get A Bivalent Covid 19 Booster Johns Hopkins Bloomberg School Of Public Health
Covid 19 Bivalent Vaccine Boosters Medical Health Cluster A C

Opinion Don T Skip Your Covid Bivalent Booster Shot The Washington Post
Bivalent Covid 19 Booster Vaccine Approved In Uk European Pharmaceutical Manufacturer
New Covid Bivalent Vaccine Expected In Us In The Fall Abc News
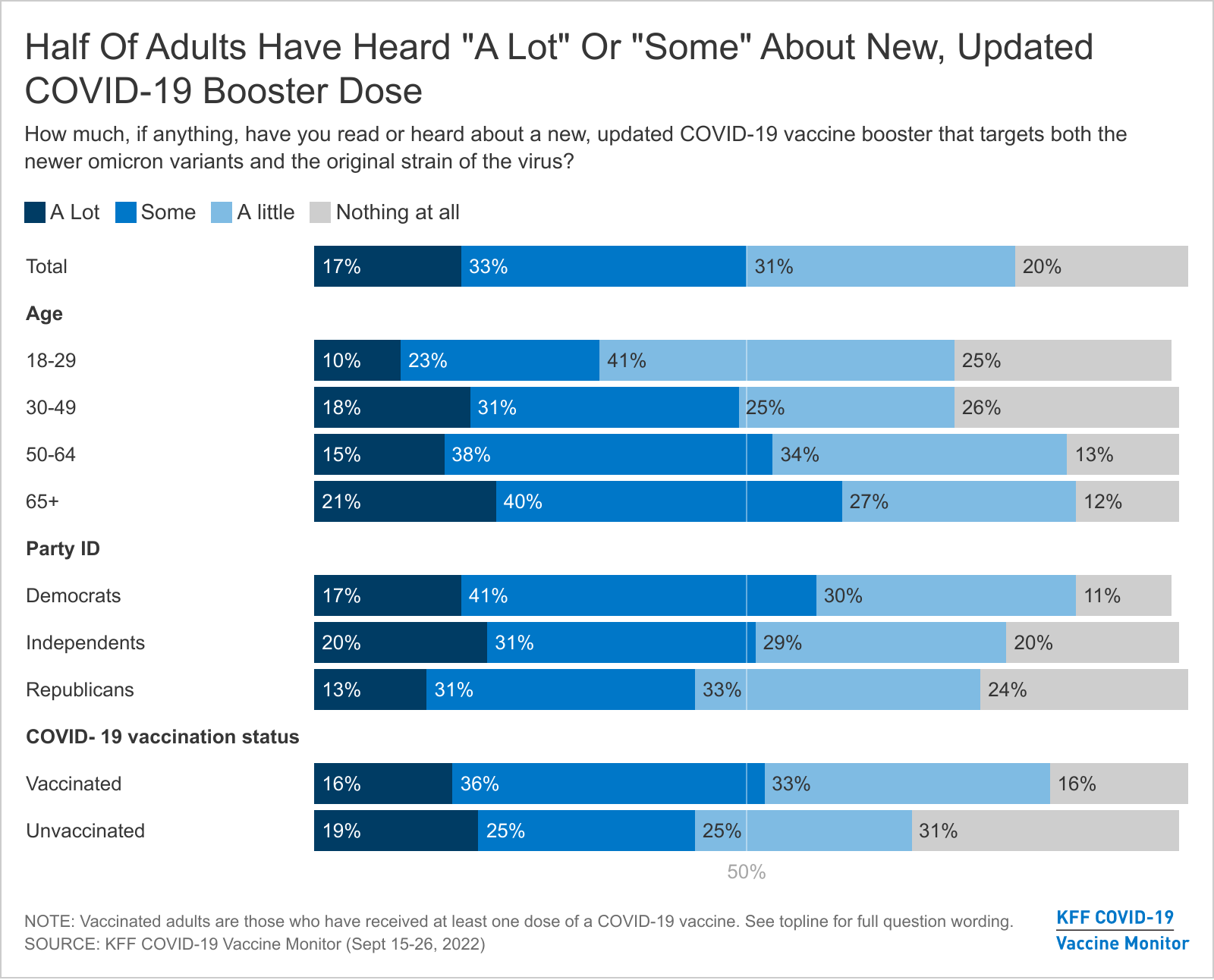
Half Of Public Has Heard Little Or Nothing About The New Covid 19 Booster Aimed At Omicron Many Don T Know If The Cdc Recommends That They Get The New Booster Kff

Moderna Says Its New Bivalent Vaccine Shows Promise Shots Health News Npr
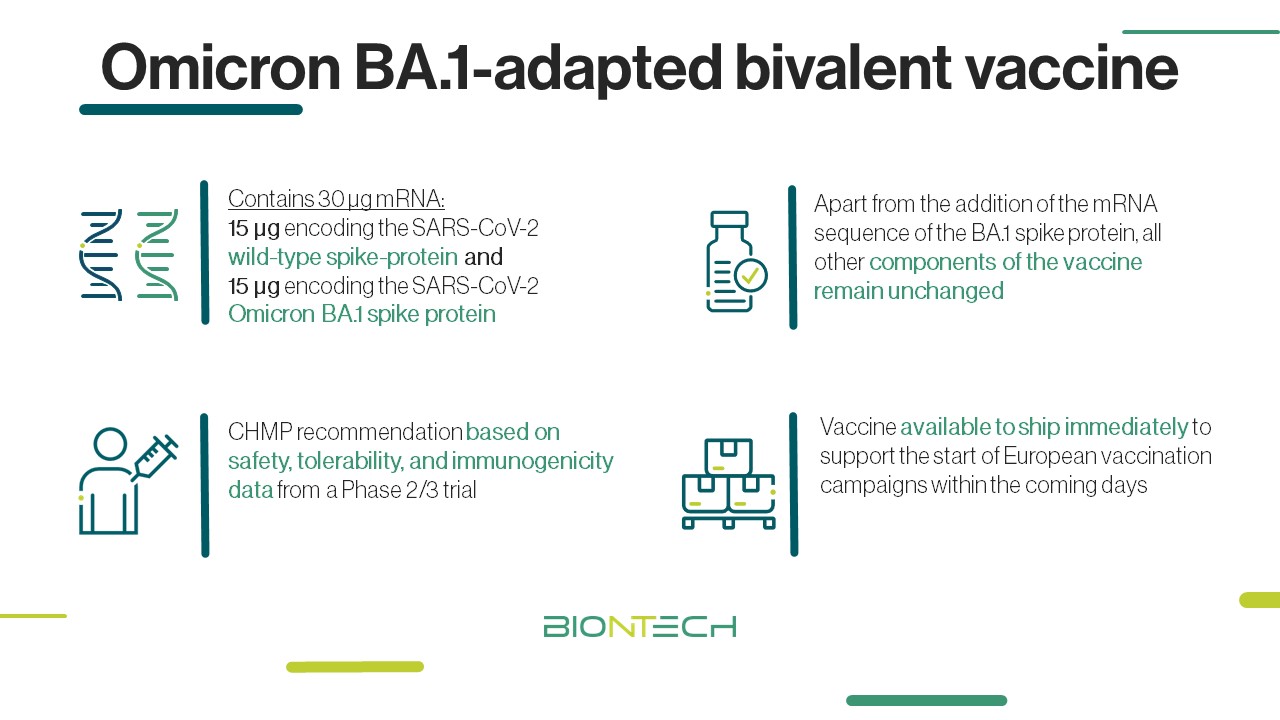
Biontech Se On Twitter Omicron Ba 1 Adapted Bivalent Vaccine Update Our Ba 1 Bivalent Covid 19 Vaccine Was Recommended For Conditional Marketing Authorization By Ema News Committee For Medicinal Products For Human Use As A Booster

A Bivalent Omicron Containing Booster Vaccine Against Covid 19 Nejm

Health Canada Approves Moderna S Covid 19 Vaccine For Omicron Ba 4 Ba 5 Variants Cbc News

Bivalent Conjugate Vaccine Induces Dual Immunogenic Response That Attenuates Heroin And Fentanyl Effects In Mice Bioconjugate Chemistry
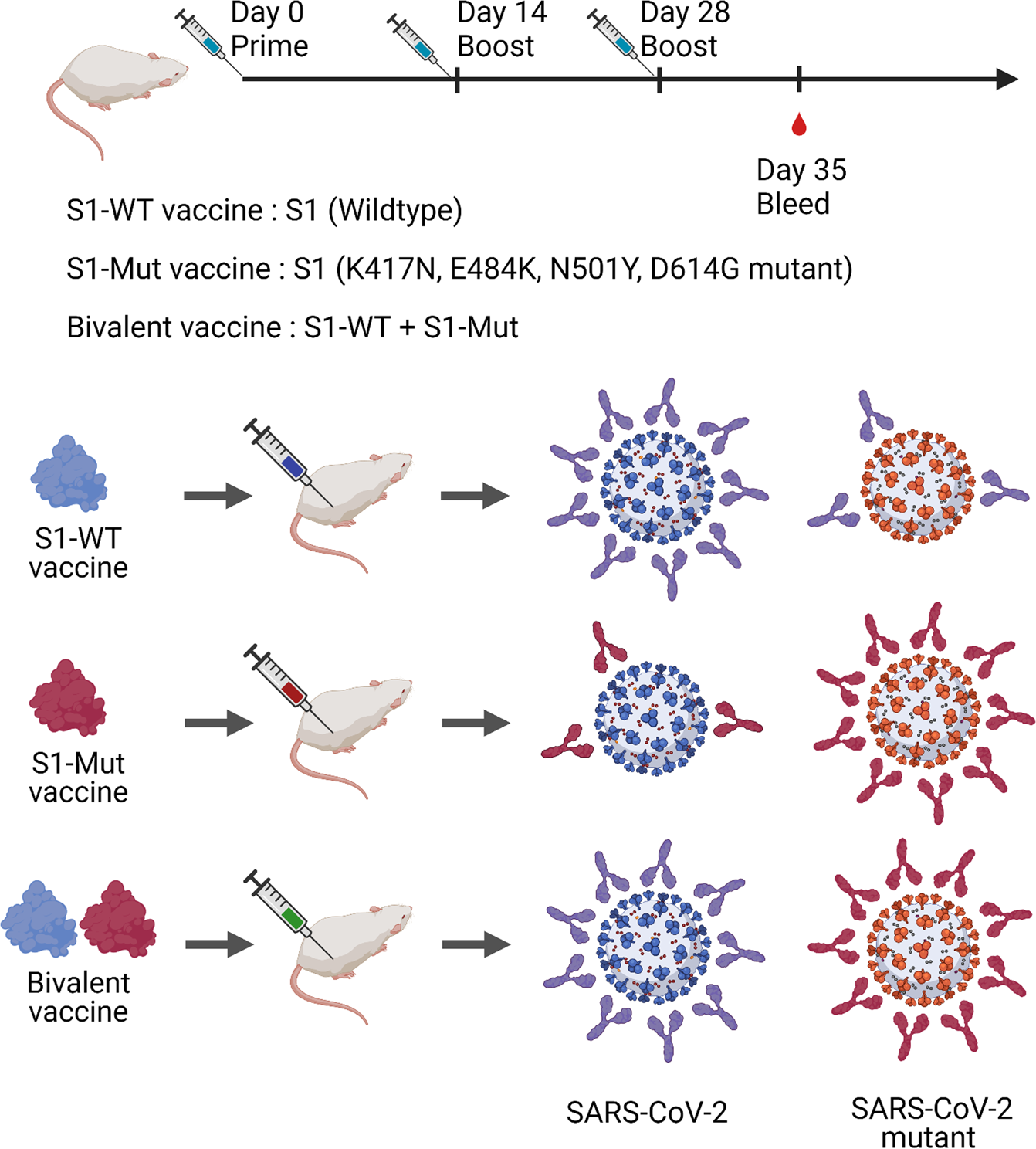
A Bivalent Recombinant Vaccine A Promising Strategy Against Both Sars Cov 2 Variants And Wild Type Of The Virus Signal Transduction And Targeted Therapy

Covid Vaccines And Boosters Tricare

Fda Panel Gives Thumbs Up To Omicron Containing Covid Boosters Medpage Today
What You Need To Know About Bivalent Boosters For Covid 19 Hub
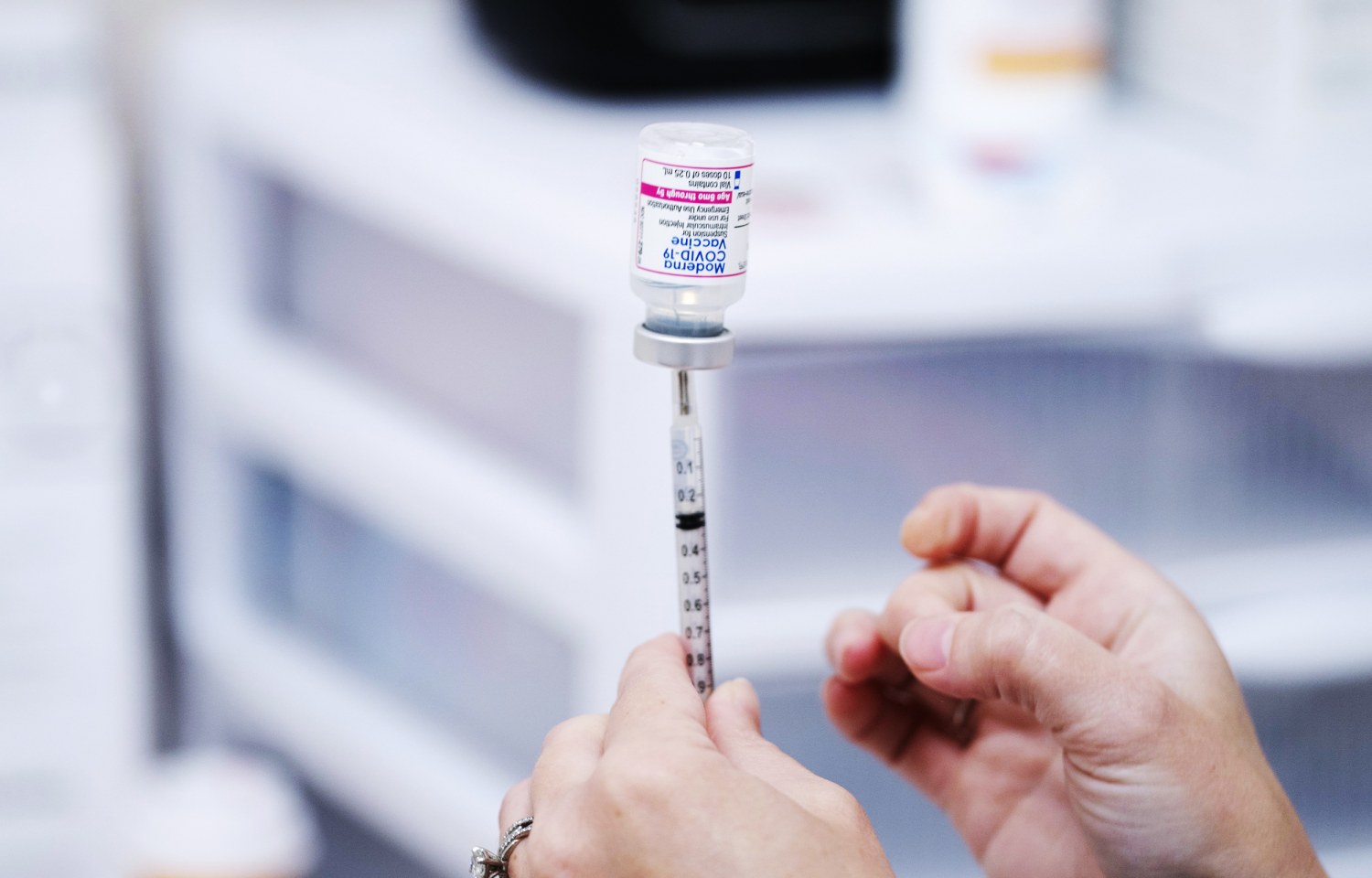
Moderna Bivalent Covid Vaccine Appears To Work Against Omicron Subvariants Ba 4 And Ba 5

British Regulator 1st To Authorize Moderna S Updated Covid Booster Cbc News